Iodine Test for Starch
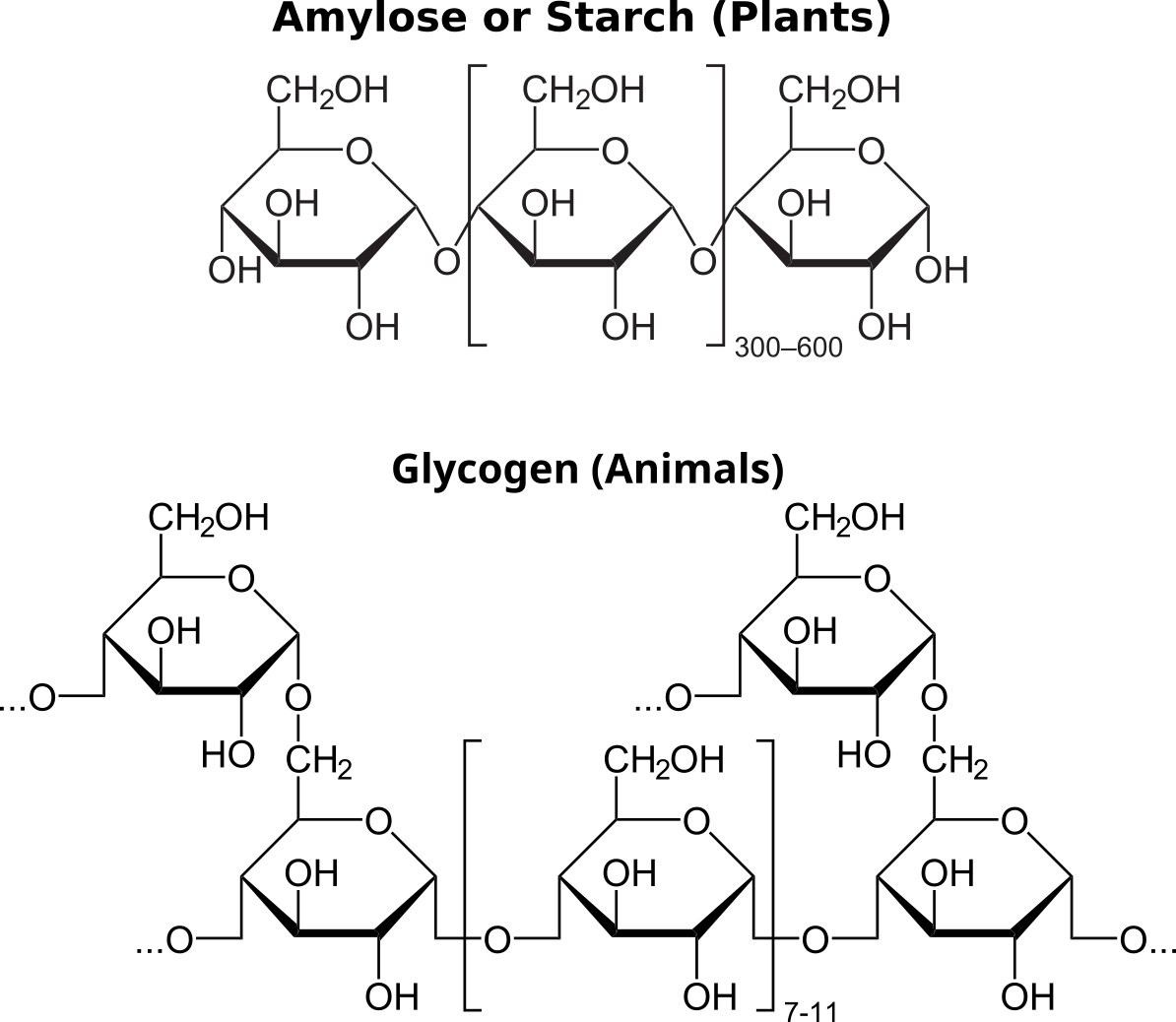
Plants store carbohydrates as a simple repeating polymer of glucose called starch. Amylose is a type of starch. Animal cells store glucose into a storage polymer called glycogen which is slightly more complicated than amylose.
Carbohydrates that are used for energy storage are not reducing sugars since they are polymers that lack free aldehydes. Plant cells store energy in the form of starches like amylose or pectin. Since these molecules are larger than monosaccharides or disaccharides, they are not sweet to the taste and are not very soluble in water. Iodine (iodine-potassium iodide, I2KI) staining distinguishes starch from monosaccharides, disaccharides, and other polysaccharides. The basis for this test is that starch is a coiled polymer of glucose — iodine interacts with these coiled molecules and becomes bluish black. Iodine does not react with other carbohydrates that are not coiled, and remains yellowish brown. Therefore, a bluish black color is a positive test for starch, and a yellowish brown color (i.e., no color change) is a negative test for starch. Notably, glycogen, a common energy storage polysaccharide in animals, has a slightly different structure than does starch and produces only an intermediate color reaction.
Activity: Iodine Test For Starch
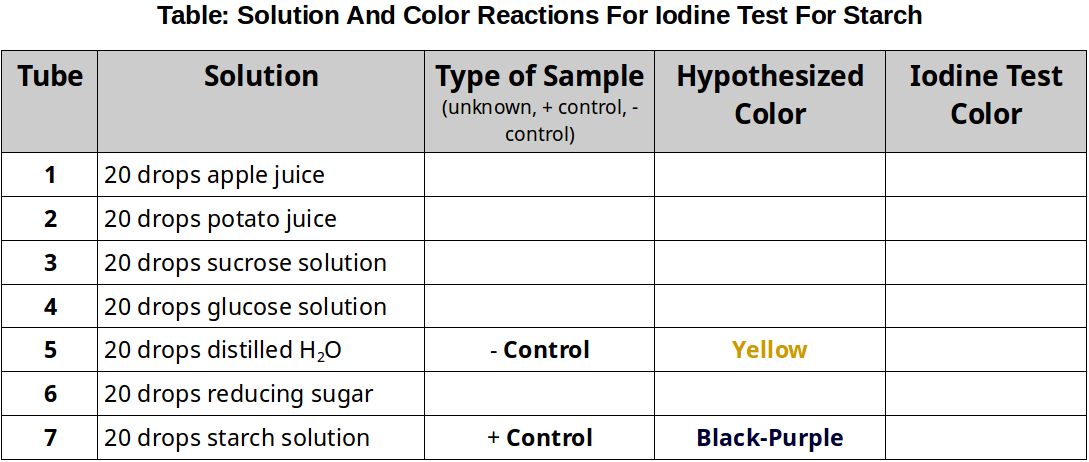
- Obtain 7 test-tubes and number them 1-7.
- Hypothesis Testing: Indicate in the table if the sample is experimental or control. Predict your expected color changes for each sample.
- Add to each tube the materials to be tested as indicated in the table below. Your instructor may ask you to test some additional materials. If so, include additional numbered test tubes.
- Add 10 drops of iodine to each tube. This test does NOT require boiling.
- Record the color of the tubes’ contents in the table below.
Questions for Reflection
-
- In the Benedict’s test, which of the solutions is a positive control? Which is a negative control?
- Which is a reducing sugar, sucrose or glucose?
- Which contains more reducing sugars, potato juice or onion juice?
- Is there a difference between the storage of sugars in onions and potatoes?
- Which patient sample likely comes from a diabetic patient and how do we know this?
- In the Iodine test, which of the solutions is a positive control? Which is a negative control?
- Which is more positive for the iodine test: onion juice or potato juice?
- What can you infer about the storage of carbohydrates in onions? In potatoes?
- Describe the half reaction Cu2+ → Cu+ as oxidation or reduction.
- Describe the half reaction Cu+ →Cu as oxidation or reduction.

