Contents
Specific Learning Objectives:
After doing this lab students will:
- Articulate the function of cellular respiration and identify the reactants and products of this reaction.
- Describe the role of enzymes on the rate of cellular respiration.
- Measure the effect of temperature and other factors on cellular respiration.
- Explain the differences between anaerobic fermentation and aerobic cell respiration.
- Identify the presence of CO2 as a product in the cellular respiration and fermentation.
- Identify using of sugar as fermentation substrate in fermentation.
- Identify the presence of alcohol as a product of yeast fermentation process,
- Evaluate the correlation between the amount of released CO2 and decreasing pH.
- Interpret the effect of the rate of the cellular respiration on global climate change
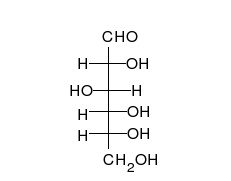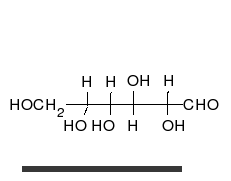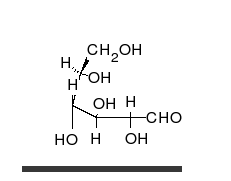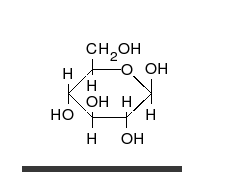
Glucose is the preferred carbohydrate of cells. In solution, it can change from a linear chain to a ring.
Glycolysis
Glucose is the preferred carbohydrate of cells. Glycolysis (glyco – sugar; lysis – splitting) is a universal process of all cells that occurs in the cytosol whereby the glucose (a 6-carbon sugar) is split into two pyruvate (a 3-carbon molecule) molecules to generate ATP and reduced NADH. ATP (adenosine triphosphate) is the energy currency of the cell that stores chemical energy in 3 high energy phosphate bonds. NADH (reduced nicotinamide adenine dinucleotide) is a high energy electron carrier that acts as a coenzyme in reactions and as a rechargeable battery of sorts. The uncharged state that is not carrying high energy electrons is called NAD+.

Glycolysis is the splitting of glucose into 2 pyruvate molecules to generate 2 NADH and 2ATP molecules.

NADH is the reduced form of NAD+. The High energy electrons associated with the reduced form come with a H atom.
Fermentation
In the absence of oxygen, cells may decide to utilize the pyruvate from glycolysis to rapidly generate additional ATP molecules in a process called fermentation. Fermentation is the anaerobic process of reducing pyruvate to generate ATP. This process uses the NADH generated from glycolysis as the reducing agents. Fermentation is a familiar process that occurs in yeast to generate ethanol. In other organisms, like humans, fermentation results in the production of lactic acid. Both lactic acid and ethanol are toxic, but this aids the cells in generating ATP when energy is required rapidly. Fermentation also generates CO2 as a waste molecule as pyruvate is broken down into a 2-carbon compound.
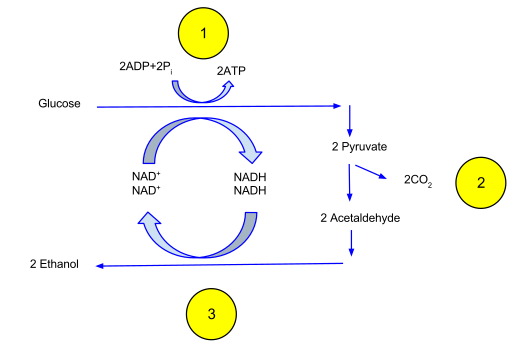
Fermentation in yeast generates ATP in the absence of oxygen but yields little ATP at the cost of the reduced NADH. Credit: Davidcarmack (CC-BY-SA)
The Preparatory Reaction
In the presence of O2, aerobic organisms will use a reaction of pyruvate decarboxylation in the cytosol. This reaction generates a molecule of Acetyl-CoA from the Coenzyme A which can enter the mitochondria.

Coenzyme A (CoA) is charged with an Acetyl group (2 carbon compound) to generate Acetyl-CoA and a CO2.
When there is an excess of carbohydrates, the Acetyl-CoA is used as a starting point for long-term energy storage in lipid synthesis.
Mitochondria
Mitochondria are the power station of eukaryotic cells. They are derived from a process described by the endosymbiotic theory whereby aerobic prokaryotes were engulfed by a protoeukaryote. In this mutualistic arrangement, the prokaryote detoxified the deadly O2 gas in the environment and used it to fully break down glucose to yield many ATP molecules. Evidence for this theory comes from the independent replication of the mitochondria, the bacterial-like mitochondrial DNA, the bacterial-like mitochondrial ribosomes, the bacterial lipids found in the inner membrane and the eukaryotic nature of the outer membrane. Mitochondria are genomically similar to bacteria of the order Rickettsiales. Some bacteria of this order are still free-living and some are intracellular pathogens.

Credit: Kelvinsong (CC-BY-SA 3.0)
Aerobic Respiration

Cellular Respiration. Left side is glycolysis (anaerobic). The Right side is what occurs in the presence of oxygen in eukaryotes. The aerobic reactions occur inside the mitochondria after being fed Acetyl-CoA molecules from the cytoplasmic preparatory reaction. Credit: RegisFrey (CC-BY-SA 3.0)

Closeup of the Electron Transport Chain (ETC) that takes place on the inner membrane of mitochondria. This is where oxygen is utilized as the final electron acceptor. Reduction of 1/2 O2 results in the generation of a water molecule (chemiosmosis). Credit: Jeremy Seto (CC-BY-NC-SA 3.0)
Metabolic Pool
The catabolic pathways involved in the glycolysis and the Krebs cycle constitute the metabolic pool that supplies building blocks for other anabolic reactions in the cell. An excess of carbohydrates can result in an accumulation of Acetyl-CoA molecules. If there is a great excess of Acetyl-CoA, the acetyl groups can be committed to fatty acid synthesis for long-term energy storage. Glycolytic products can also be the starting point for amino acid synthesis. 3-phosphoglycerate can be used to synthesize glycine, cysteine and serine. Pyruvate can be used to generate alanine, valine and leucine. Oxaloacetate from the Krebs cycle can be used as a starting point for aspartate, lysine, asparagine, methionine, threonine and isoleucine. Glutamate and glutamine are synthesized from α-ketoglutarate formed during the Krebs cycle. While most of the 20 amino acids can be synthesized de novo, there are 9 essential amino acids in humans that can not be synthesized in sufficient quantity and therefore must be gained from the diet. These essential amino acids include: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine.



