Activity: Do larger things diffuse faster?
Agar is a gelatinous substance derived from a structural carbohydrate found in seaweed. It is often used in cooking as a vegetarian alternative to gelatin and can be used as a thickener. Microbiologists pour plates of agar containing nutrients in order to isolate and grow bacteria and other microbes. As with gelatin, the long fibery nature of this structural carbohydrate permits it to be melted and tangled together in a mesh-like network where the spaces between molecules are filled with solution. Altering the amount of fluid solution will change the pores between fibers. More fluid will create a looser gel that has larger spaces between molecules. Reducing the fluid solution volume will conversely create a stiffer gel with smaller spaces between fibers.
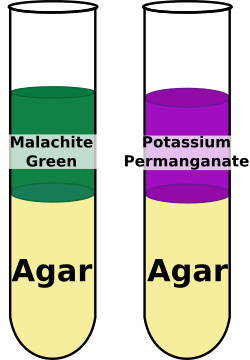
- Take 2 tubes of agar and a solution of Malachite green (365 g/mole) and a solution of Potassium permanganate (164 g/mole)
- Mark the top of the agar on the outside of the tube (the starting point)
- Add 10 drops of malachite green to one tube and 10 drops of Potassium permanganate to the other
- Take note of the time
- At 20 minute intervals, measure the distance from the top that the agar has moved. Do this for at least 1 hour.
- Plot the data and compare the trends. Describe the rate of diffusion for each.
Stop and Think:
- Hypothesize which solution will move faster through the agar and provide a reason.
| Diffusion speed of dye molecules | ||||
|---|---|---|---|---|
| Dye |
Molecular Weight |
Hypothesis (fast or slow diffusion) |
||
| Malachite Green |
365 g/mole |
|||
| Potassium Permanganate |
164 g/mole |
|||
Conclude:
- Which solution actually moved faster?
- Did this meet your expectations?
- Propose a reason why a certain dye moved faster.
- Review through simulation