Investigating Photosynthesis using Algal Balls
Overview:
This lab is designed as a flipped lesson to provide introductory biology students with a basic understanding of photosynthetic pathways and how environmental factors affect the photosynthetic rate in autotroph living organisms such as plants and algae.
Therefore, Students need to watch the provided short videos in each concept and answer the related questions before coming to the lab in order to be ready to do the experiments in the lab.
Contents
Learning Objectives/Outcomes
Students will understand the process of photosynthesis in plant and algae and different environmental factors that would affect the rate of photosynthesis. They will also appreciate the biological processes associated with dead zones, as well as how human activities impact the severity of dead zones. Students will also collect and plot data and use scientific equipment.
After completing this lab students will:
- Define the structure of a plant leaf.
- Asses the number of stomata in provided leaves from different areas of NYC.
- Interpret why plant leaves appear to be green.
- List and describe the process of photosynthesis.
- Interpret the presence of starch in a plant leaf.
- Describe why algae are able to make their own food.
- Report how different colors of visible light affect photosynthetic rate.
- Report how CO2 concentration and elevated temperature affect photosynthetic rate.
- Report how the fertilizer and Herbicide affect photosynthetic rate.
- Describe the relationship between Photosynthesis and Cellular respiration.
- Explain how photosynthesis benefits human.
- Apply their knowledge from doing experiments in this lab to explain how dead zones would form in oceans and how this process could affect the human life and ecosystem in general.
- Apply their knowledge from doing experiments in this lab to explain how climate change would affect human life and ecosystem in general.
Pre-Lab Assignments:
Concept 1: An overview of Photosynthesis
Photosynthesis is a process that converts carbon dioxide into sugars such as glucose using energy from the sun. When light is absorbed by pigments in a leaf, the energy absorbed is used to incorporate the carbon dioxide into organic molecules in a process called carbon fixation.
The process of photosynthesis can be expressed by the following word equation and chemical equation.
Carbon dioxide + Water → Glucose + Oxygen
6CO2 + 12H2O → C6H12O6 + 6O2 + 6H2O
Go to the “What is Photosynthesis” page by clicking on the link:
https://ssec.si.edu/stemvisions-blog/what-photosynthesis
In this page you will be familiar with an overview of photosynthesis and its importance in human life and ecosystem. At the end of this page watch “Photosynthesis: Blinded by the Light” to explore some misconceptions about matter and energy in photosynthesis and strategies for eliciting student ideas to address or build on them.
Adapted from: This is an excerpt from the Structure and Function unit of our curriculum product line, Science and Technology ConceptsTM (STC).
Answer the following questions.
- Besides CO2, ___________ is also used in photosynthesis to produce _____________ molecules such as the sugar _____________.
- Living organisms such as plants that make their own food are called______________.
- Explain how does a pea plant grow larger?
- Explain how is photosynthesis important for the animals lives?
- Sketch a diagram that shows an overview of photosynthesis
Concept 2: Electromagnetic Energy
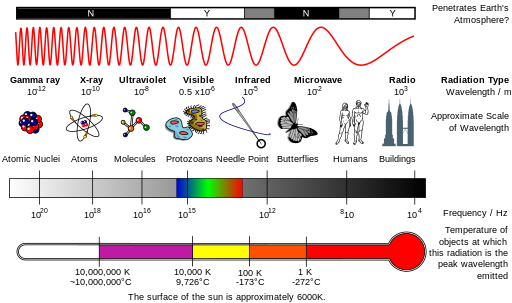
The solar energy called visible light drives photosynthesis. Solar radiation is composed of electromagnetic energy that travels through space in a manner analogous to the motion of waves in water. The distance between the crests of waves is called the wavelength. The shorter the wavelength, the greater the energy for each unit (photon) of electromagnetic energy. Remember, energy cannot be created or destroyed. When light is absorbed by a green plant, a small portion of that energy is converted into chemical energy in the process of photosynthesis.
Watch these videos and answer the following questions.
- Light reactions-Photosynthesis
In this video you will learn why most leaves are green.
1- What is the electromagnetic spectrum?
2- What is the range of wavelength for visible light?
3- What structure in the leaves is responsible for making the leave’s color?
4- What are the different types of the pigments?
5- Why are leaves green?
- Why Do Leaves Change Color in The Fall?
In this video you will learn why green leaves will turn orange or red in fall.
1- Why do leaves change their color from green into reds, oranges, yellows, and browns in fall?
2- How would climate change affect the color of the plants at different seasons?
Concept 3: The structure of leaf
The structure of leaves promotes photosynthesis and allows the control of gas exchange and water loss.
Watch these videos and answer the following questions.
- Structure of a leaf
In this video you will learn the parts of the leaf and the structures that are responsible for photosynthesis.
1- Label the indicated structures of a leaf in this diagram

2- What is the function of stomata?
3- What is the function of cuticle?
4- Name two gases exchanged through the stomata of a leaf?
- Chloroplast-Structure
In this video you will learn the ultrastructure of the chloroplast and the function of each structure in the chloroplast.
- Sketch and label a chloroplast.
- Is the numbers of the chloroplast the same between different plants?
- Sketch and label a chloroplast.
- What is the difference between the outer and inner membrane of chloroplast?
- What are the photosystem I and II?
- Where do photosystems locate in the chloroplast?
Concept 4: The two stages of photosynthesis
Photosynthesis transfers electrons from water to energy-poor CO2 molecules, forming energy-rich sugar molecules. This electron transfer is an example of an oxidation-reduction process: the water is oxidized (loses electrons) and the CO2 is reduced (gains electrons). Photosynthesis uses light energy to drive the electrons from water to their more energetic states in the sugar products, thus converting solar energy into chemical energy.
Photosynthesis occurs in two metabolic stages.
- The light reactions occur in the thylakoid membranes and converts the solar energy to chemical energy.
- The Calvin cycle occurs in the stroma of the chloroplast that reduces CO2 to sugar during a series of chemical reactions.
Watch this animation and answer the following questions.
- The process of photosynthesis
In this animation you will be familiar with the details of the two stages of photosynthesis.
https://www.youtube.com/watch?v=1Dn_zdAZN0I
- Plants store ___________ in the chemical bonds of ____________.
- Chemical ___________ is released from sugars during _____________________.
- Name the first part of photosynthesis.
- Light reactions use energy from the _________ to produce _______ and the energy carrier _______.
- The second part of photosynthesis is called the __________ cycle.
- Does the Calvin cycle require light energy?
- The Calvin cycle is also called the __________ fixation or the ______ pathway.
- Where do the light reactions of photosynthesis take place in a chloroplast?
- The cyclic electron flow only uses ______________ I in the thylakoid membranes and has chlorophyll a molecules at the photosystem’s reaction center that absorbs ________ wavelength of light.
- Both photosystem I and II use the ________ transport ______ (ETC) to produce ATP.
- Water is split in ___________ , while NADPH is made in ____________.
- Oxygen made by plants comes from the splitting of ___________.
- _______________ powers ATP synthesis in plants.
- Where does chemiosmosis occur in plants?
- ATP is made using the _________________ chain and the enzyme ___________.
- ___________ ions move through down their concentration gradient through the ______________ of the enzyme ATP _______________ to form ATP from _________.
- ________ is the energy molecule used by cells and must be remade by ATP ___________ or chemiosmosis
Lab Activities
Investigation I: Making Leaf Impression slide:
In this activity you will be examine and compare the shape and number of stomata in the leaves that are collected from different areas in NYC.
The Stomata:
In order to provide the cells of the leaf with sufficient CO2 and to allow for the escape of O2, the bottom side of the leaves contain countless openings, the stomata. Each stoma can be opened and closed by the expanding and contracting action of two guard cells. The stomata open during the day to allow for the free movement of gases into and out of the leaf. At night, when photosynthesis does not take place, the guard cells close the stomata to minimize the loss of water. Exceptions do exist, such as the CAM (Crassulacean acid metabolism) plants, which grow in hot and dry environments. These plants close the stomata during the day to further reduce the water loss. These plants then open the stomata at night to allow CO2 to enter the leaves. The CO2 is fixed and the product is stored in vacuoles to be used for photosynthesis during day.
Several researches have indicated that the air pollution such as high concentration of SO2 affects the size and density of stomata (Pourkhabbaz A. et al, 2010, V. D. K. AbeyratneOliver and IleperumaOliver, 2017).
Making leaf impressions is probably one of the least complicated and easiest methods to make the surface texture and the stomata of the leaves visible. This video shows you how to take an imprint of a leaf.
https://www.youtube.com/watch?v=UqdyP_HZvPY
Procedure:
- Use clear nail polish to paint and cover two areas of about 2 cm3 on the underside of a leaf.
- Allow the nail polish dry out thoroughly for about 5 minutes to avoid denaturing of the imprint.
- Cover each of the painted areas with a piece of clear tape and fully press.
- Slowly peel off the tape.
- Place the tape on top of the clean slide
- Observe the slide under the microscope.
- Count the number of stomata in 5 different field of view by using 40X objective lens and record your data in table 1.
- Measure the widths of 5 stomata in 5 different areas by using a calibrated micrometer slide.
- The width should be recorded from the middle area of the pore, between the inside walls of guard cells which line the stomatal pore. This gives you the maximum pore width.
- Record your data in table 2.
Table 1
| Number of stomata | Class average | |
| Leaf#1 | ||
| Leaf#2 | ||
| Leaf#3 | ||
| Leaf#4 |
Table 2
| Diameter in μM | Class average | |
| Leaf#1 | ||
| Leaf#2 | ||
| Leaf#3 | ||
| Leaf#4 |
Explain your findings by comparing the data in above tables.
Investigation II: Testing a leaf for starch- Demonstration
Testing a leaf for starch is the simplest photosynthesis lab. A positive test for starch in a leaf provides evidence that photosynthesis has occurred. The test for starch is a leaf is an is an extension of the Iodine Test for Starch.
Glucose is the product of photosynthesis, and is rapidly converted to granules of starch – a polymer of glucose – for storage. Starch granules have been visualized in the stroma of the chloroplast as well as the cytoplasm.
Procedure:
- Remove a green leaf from a plant that has been exposed to sunlight for a few hours
- Half-fill a 250cm3 beaker with water. Heat the water until it boils. Keep the water at boiling point.
- Use the forceps to place the leaf in the boiling water. Boil for 2 minutes.
- Turn off the Bunsen Burner.
- Place the boiled leaf in a boiling tube containing 90% ethanol.
- Place the boiling tube in hot water and boil for 10 minutes or until the leaf decolourizes.
- Gently remove the leaf and wash with cold tap water.
- Spread the leaf evenly on a white depression plate.
- Add a few drops of iodine/potassium iodide solution to the leaf and record your observations.
The color of iodine solution before adding to the leaf: _________________
The color of iodine solution after adding to the leaf: ___________________
Interpretation:
Which products of photosynthesis may be present but not revealed by the iodine test?
Investigation III: Chlorophyll Chromatography-Demonstration
Chlorophyll is the green pigment responsible for the color of leaves. Its presence in leaves is crucial for photosynthesis. Photosynthesis can be defined as the process by which plants, algae, and photosynthetic bacteria use light energy to drive the synthesis of organic compounds. The photosynthetic process involves the removal of CO2 from the atmosphere, which is used to synthesize carbohydrates, and results in the release of O2. The energy to drive the chemical reactions of photosynthesis comes from the sunlight absorbed by the chlorophyll molecules. Hence, the first step in photosynthesis is the absorption of visible light from the sun by chlorophyll molecules. The chlorophyll molecules then transfer the light energy to chloroplasts, the reaction center of photosynthesis. In this way light energy is converted to chemical energy for converting CO2 into carbohydrates.
In this video you will learn the procedure of extraction Chlorophyll.
Investigation IV: Investigating factors affecting the rate of photosynthesis using immobilized algae
In this activity, you will use algae to look at the rate of photosynthesis. Since algae are tiny and are difficult to work with directly in the water, the first part of the practical involves ‘immobilizing’ the algae as algal balls. This effectively traps large numbers of algal cells in ‘jelly like’ balls made of sodium alginate. Sodium alginate is not harmful to the algae, and they will continue to photosynthesis once immobilized.
When these algae are ‘wrapped up’ in the jelly balls they are excellent to use in experiments on photosynthesis.
This procedure offers a method for measuring the rate of photosynthesis which depends directly on the rate of uptake of carbon dioxide by the photosynthetic organism. Hydrogencarbonate indicator changes color with pH, which is determined by the concentration of carbon dioxide in solution.
There is scope for students to develop the protocol to investigate a range of factors.
This protocol is adapted with permission from information on the Science and Plants for Schools (SAPS) website (see www.saps.org.uk).
This video shows you the procedure of making algal balls
This procedure gives you a chance to:
- learn how to trap algae in alginate beads
- evaluate this method for investigating photosynthesis and plan your own investigations.
Procedure
SAFETY: Take care with the very bright lights needed for this investigation. Do not look directly into them. Be aware they may be hot, so handle with care and keep them away from anything flammable.
Investigation
Making your algal beads
a Concentrate your active algal culture, by leaving 50 cm3 to settle for 30 minutes in a measuring cylinder until you have a darker green sediment. Carefully pour off the pale green suspension to leave just 5 cm3 of concentrated culture. This may have been done for you.
b Mix 5 cm3 of algal culture with 5 cm3 of 3% sodium alginate solution in a very small beaker with a cocktail stick.
c Clamp a syringe barrel above a beaker of calcium chloride solution – making sure the tip of the syringe is well above the solution in the beaker. See diagram.
d Pour the alga/ alginate mixture through the syringe so it drips through and forms beads in the beaker. Swirl the beaker gently as the drops fall.
If your solution is too thick (viscous) to drip through, dilute it with a little more algal suspension or pure water and try again. If it is running through too quickly, mix in some more alginate.
e Allow the beads to harden for a few minutes before straining them out of the beaker through a tea strainer.
f Rinse the beads in distilled water. The algae in the beads will stay alive for several weeks if they are kept in a stoppered bottle of distilled water in a refrigerator.
Investigating the effect of light in the rate of photosynthesis
Result Predications
| Time | Predictions | ||||
| Algae/ Light | Algae /Dark | Water/ Light | Water/ Dark | ||
| 30 min | Some of the CO2will be converted to glucose, which will decrease the amount of carbonic acid, and thus the pH will increase, and the color of the indicator solution will begin to change from yellow to orange or red. | Light is a component of the photosynthetic reaction and it cannot occur without. The amount of carbonic acid will stay the same, no color change. | The CO2 will NOT convert to glucose, the amount of carbonic acid will remain the same, and thus the pH will remain the same and there will be no color change. | ||
| 24 hours | Most of the CO2 will be converted to glucose and the color of the indicator solution will be purple. | ||||
| pH Color Standards – Recipe Chart | |||||||||
| pH | 7.6 | 7.8 | 8.0 | 8.3 | 8.4 | 8.6 | 8.8 | 9.0 | 9.2 |
| Boric Acid | 25 mL | 25 mL | 25 mL | 25 mL | 25 mL | 25 mL | 25 mL | 25 mL | 25 mL |
| Borax | 1.0 mL | 1.55 mL | 2.45 mL | 3.8 mL | 5.7 mL | 8.7 mL | 15.0 mL | 29.5 mL | 57.5 mL |
| Add enough dH2O to bring the total volume in each container to 100 mL | |||||||||
Investigating the effect of light intensity in the rate of photosynthesis
| pH Color Standards – Recipe Chart (2.0 mL tubes) | |||||||||
| pH | 7.6 | 7.8 | 8.0 | 8.3 | 8.4 | 8.6 | 8.8 | 9.0 | 9.2 |
| Boric Acid | 500 μL | 500 μL | 500 μL | 500 μL | 500 μL | 500 μL | 500 μL | 500 μL | 500 μL |
| Borax | 20 μL | 31 μL | 49 μL | 72 μL | 114 μL | 174 μL | 300 μL | 590 μL | 1150 μL |
| 10X indicator | 200 μL | 200 μL | 200 μL | 200 μL | 200 μL | 200 μL | 200 μL | 200 μL | 200 μL |
| Add enough dH2O to bring the total volume in each container to 2 mL | |||||||||
- Investigating the effect of environmental factors on the rate of photosynthesis
- Color filters or light bulbs of different colors provide a simple but powerful illustration of the absorption properties of photosynthetic pigments. Each filter transmits the color that it appears to our eyes and absorbs other visible wavelengths to varying degrees. Ask your students to consider the absorption spectrum of chlorophyll and minor photosynthetic pigments. Which filter should allow the greatest activity? the least?
- Gather red, blue, green, and transparent color filters. Which is your experimental control?
- Add 35 algae balls to each of four vials and 35 H2O/alginate balls to another vial. With which sample will you compare your H2O control?
- Prepare 20 mL of 1X hydrocarbonate indicator solution. Dispense 3.0 mL to each vial. Invert the vial gently and allow the solution to equilibrate for 3 minutes.
- Determine the initial pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, transfer 1.5 mL from each vial to a cuvette for an initial absorbance reading. Be sure to keep track of the sample to which each cuvette corresponds. Then re-pour the solution from the cuvette back into its corresponding sample vial.
- For each filter setup, place the vial at an angle into a paper cup. Sit the filter over the mouth of the cup.
- Shine a CFL bulb over all setups. Be sure that all vials are equidistant from the light source.
- Record the final pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, remove 1.5 mL of the indicator solution from each vial and transfer to a cuvette. Be sure to keep track of which sample each cuvette corresponds to. Record the absorbance of each sample.
| Sample | abs at time 0 (absinitial) | abs at 30 minutes (absfinal) |
| H2O/alginate control | ||
| transparent filter | ||
| red filter/light | ||
| green filter/light | ||
| blue filter/light |
ii- External carbon sources test the ability of carbohydrates to feedback inhibit enzymes required for photosynthesis. The glyceraldehyde-3-phosphate produced in the Calvin-Benson cycle has two fates: conversion into starch or into the disaccharide sucrose, which subsequently is hydrolyzed into glucose and fructose. Ask your students to hypothesize how external carbon sources affect photosynthesis rate. They can compare equal concentrations of two different sugars, such as sucrose and glucose, or varying concentrations of a single sugar. The following protocol was optimized for the latter investigation, but can be modified to test equimolar glucose and sucrose.
- Prepare 9 mL of 0.5M glucose and 9 mL of 0.1M glucose. Add 1 mL 10X hydrocarbonate indicator solution to each.
- To 18 mL distilled H2O, add 2 mL 10X hydrocarbonate indicator solution.
- Label each of three vials –glucose, 0.1M glucose, and 0.5M glucose. Add 35 algae balls to each. Add 3.0 mL 1X indicator solution to the –glucose vial and 3.0 mL of the corresponding solutions into the glucose vials.
- Add 35 H2O/alginate balls to a fourth vial. Label it H2O/alginate. Dispense 3.0 mL 1X indicator solution into this vial.
- Invert all vials gently and allow to equilibrate for 3 minutes.
- Determine the initial pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, transfer 1.5 mL from each vial to a cuvette for an initial absorbance reading. Be sure to keep track of the sample to which each cuvette corresponds. Then re-pour the solution from the cuvette back into its corresponding sample vial.
- Place all vial equidistant from the light source for 30 minutes.
- Record the final pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, remove 1.5 mL of the indicator solution from each vial and transfer to a cuvette. Be sure to keep track of which sample each cuvette corresponds to. Record the absorbance of each sample.
| Sample | abs at time 0 (absinitial) | abs at 30 minutes (absfinal) |
| H2O/alginate control | ||
| – glucose algae control | ||
| 0.5 M glucose | ||
| 0.1 M glucose |
iii. The intensity of light opens a deeper consideration of the properties of chlorophyll and can also be well quantified. Ask your students to hypothesize how photosynthetic rate changes with intensity. Have them apply the inverse square law to calculate the intensity at various distances from the light source. Will the brightest light necessarily induce the most photosynthesis? Because chlorophyll is a dye with electrons that can be promoted to higher energy states, it may react with other molecules in the cell or else absorb photons in a way that causes permanent structural damage. This phenomenon is called photo bleaching. At the other extreme, what is the lowest level of light that can still support photosynthesis?
- To 18 mL distilled H2O, add 2 mL 10X hydrocarbonate indicator solution.
- Label each of four vials 5, 10, 20, and 30 cm. Add 35 algae balls to each. Label a fifth vial H2O/alginate. Decide where you will place it and label it accordingly. Add 35 H2O balls.
- Dispense 3.0 mL of 1X indicator solution to each vial and invert gently. Allow to equilibrate for 3 minutes.
- Determine the initial pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, transfer 1.5 mL from each vial to a cuvette for an initial absorbance reading. Be sure to keep track of the sample to which each cuvette corresponds. Then re-pour the solution from the cuvette back into its corresponding sample vial.
- Place all vial equidistant from the light source for 30 minutes.
- Record the final pH:
- If comparing against color standards, estimate pH to the nearest tenth.
- If using the UV-vis, remove 1.5 mL of the indicator solution from each vial and transfer to a cuvette. Be sure to keep track of which sample each cuvette corresponds to. Record the absorbance of each sample.
| Sample | Intensity of light (W) | abs at time 0 (absinitial) | abs at 30 minutes (absfinal) |
| H2O/alginate control | |||
| 5 cm | |||
| 10 cm | |||
| 20 cm | |||
| 30 cm |
Make the graph and interpret your data
iv- Investigating the effect of fertilizer on the rate of photosynthesis
The context for the activities described here is eutrophication – an increase in nutrient concentration in waterways that can lead to algal blooms.
1- Obtain the prepared vials containing algae balls and fertilizer and hydrocarbonate indicator at different time points from your instructor.
2- Compare the size of the algal populations grown in fertilizer solutions by measuring absorbance of the algal cultures using a colorimeter.
3- Observe the cultures directly using a microscope. Compare algal growth over time by counting the algea using a haemocytometer.
| abs | Number of Algae/Growth | |||
| Control | Fertilizer | Control | Fertilizer | |
| Day 1 | ||||
| Day 2 | ||||
| Day 3 | ||||
| Day 4 | ||||
| Day 5 | ||||
Make a graph of your findings.
Interpret your findings
Watch the following videos
Climate action:
https://www.un.org/sustainabledevelopment/climate-change-2/
Climate action: Why It Matters?