Chloroplasts

Credit: Kelvinsong [CC-BY-SA 3.0]
Light Harvesting
The thylakoid membranes of chloroplasts and cyanobacteria provide additional surface area for energy capture of light to occur. The light-dependent reactions in chlorplasts utilize two protein complexes referred to as Photosystem I (PSI) and Photosystem II (PSII)located on the thylakoid membranes. At the center of each photosystem complexes are photopigments optimized to absorb specific wavelengths of light. When light is absorbed in a photosystem, an electron is excited and transferred to the electron transport chain. In PSII, the electron is regenerated by splitting of two water molecules into 4H+ + 4e– + O2. As the electrons move through the ETC, protons are pumped into the thylakoid space. The ETC leads to the reduction of a high energy electron carrier NADP+ to NADPH. Since this pathway uses consumes water in a chemical reaction, the apparent loss of water in the thylakoid space is referred to as chemiosmosis.
PSI is also known as the cyclic pathway since the excited electron runs through a closed circuit of the ETC to regenerate the lost electron. This closed circuit also generates a proton gradient through powering of a proton pump but does not lead to the reduction of NADPH. As with the ETC-powered proton pump in mitochondria, the proton gradient is used to power ATP-synthase in producing ATP molecules.
Light Independent Reactions
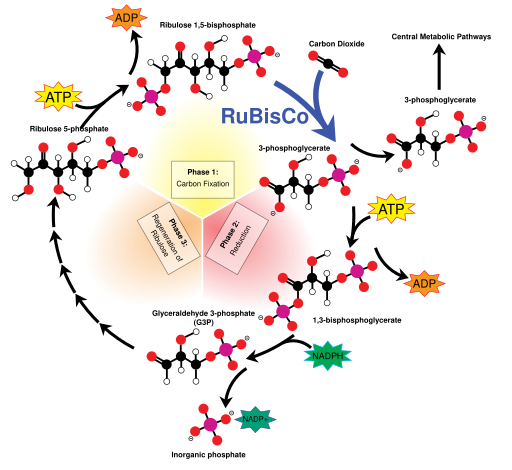
Credit: Mike Jones [CC-BY-SA 3.0]
The Great Oxygenation Event
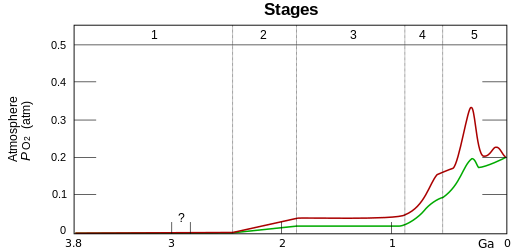
Two estimates of evolution of atmospheric O2. The upper red and lower green lines represent the range of the estimates. Stage 1 (3.85–2.45 Ga) represents the primordial reducing atmosphere. Stage 2 (2.45–1.85 Ga) coincides with the emergence of oceanic cyanobacteria where O2 was being absorbed by the oceans and sediment. O2 escaped the oceans during Stage 3 (1.85–0.85 Ga). O2 sinks filled in Stage 4 (0.85–0.54 Ga ) and Stage 5 (0.54 Ga–present) leading to atmospheric accumulation.

Banded iron formations in 2.1 billion year old rock illustrate the oxidation of dissolved oceanic iron that precipitated in response to accumulating O2 concentrations.
https://youtu.be/dO2xx-aeZ4w